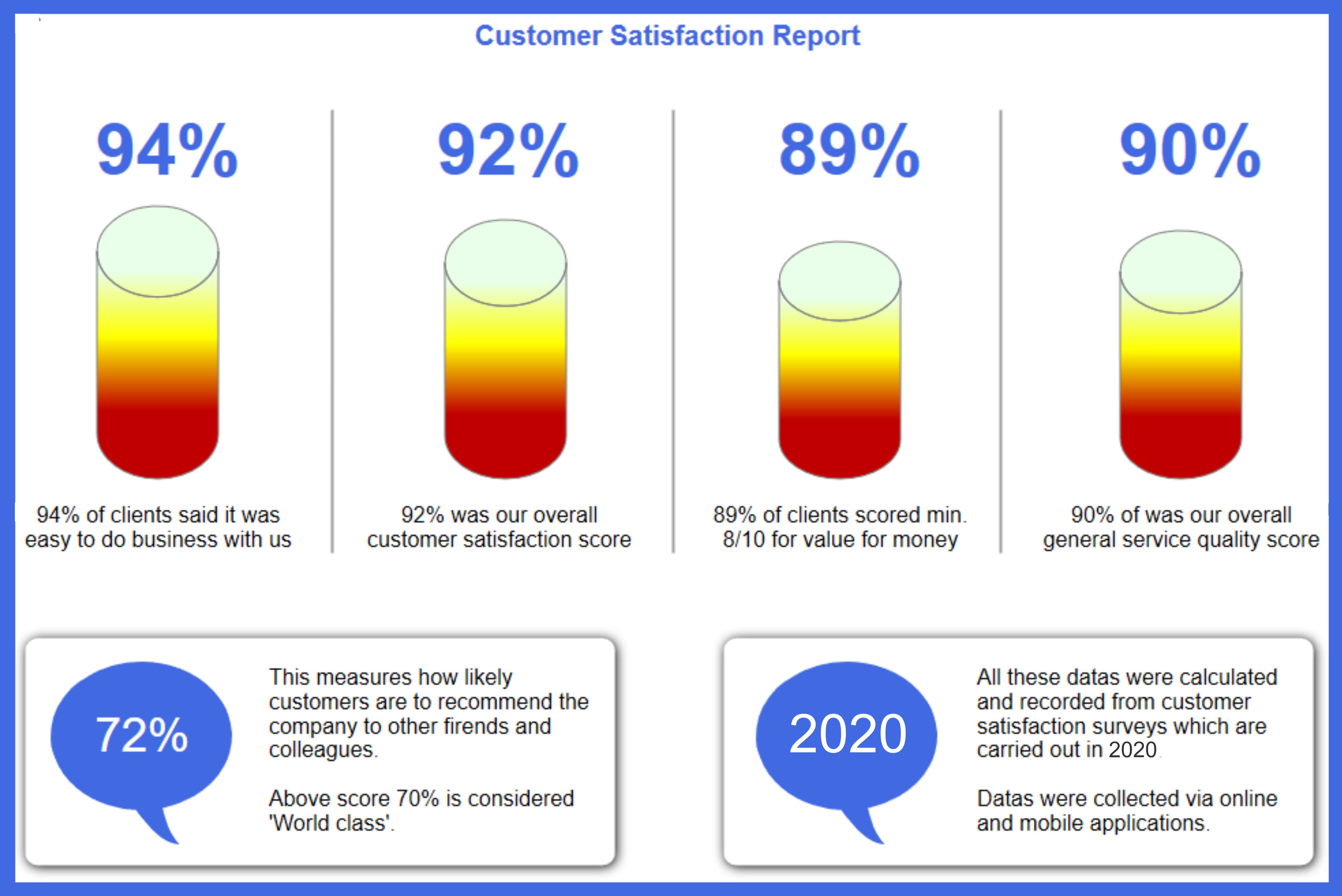
-
Your shopping cart is empty!
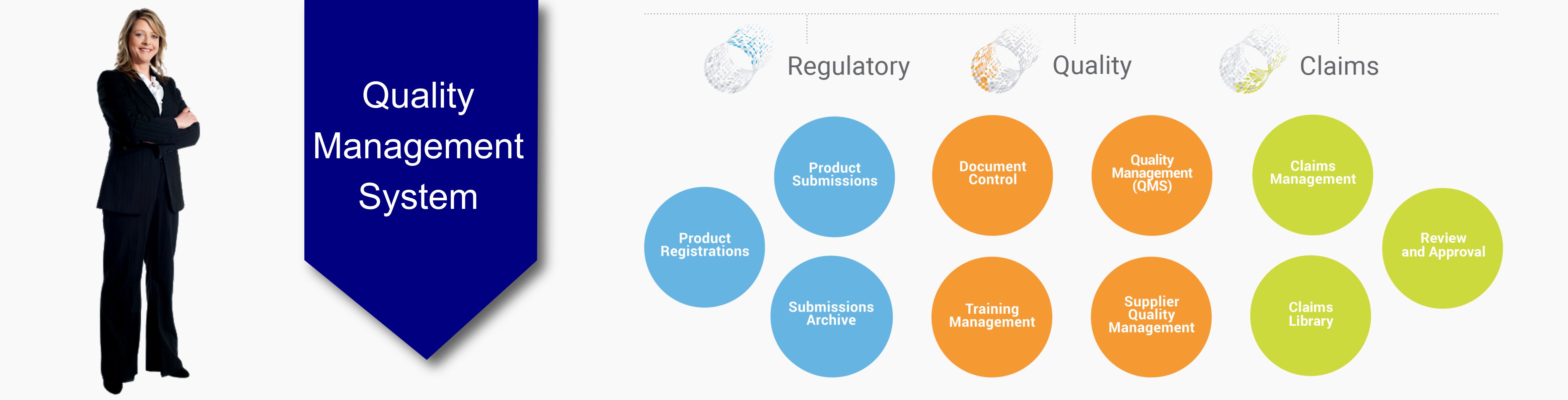
Quality
understanding is a matter handled and managed at the highest level within
Labsert. This is also a matter used as a management system not only in
manufacturing process but also in fields such as management of customer
services, shipments, human resources etc. Improvement of quality system is
carried out as a regular work in Labsert. We provide necessary resources and
carry out our operations by being aware that total quality understanding is the
fundamental source of sustainability in all activities of the company.
Quantitative analysis and tests during certification work are carried out at our sub-contracted business partnership ISO 17025 accredited laboratories and/or Labsert laboratories. As a part of manufacturing process of reference standards, detailed result of analysis is presented on a report which is prepared according to ISO 17034 assessment (Certified by Vertacert). Product certification for chemicals and sorbent materials is prepared according to ISO 9001 procedures (Certified by Vertacert).
Labsert's manufacturing capacity is registered by The Union of Chambers and Commodity Exchanges of Turkey (TOBB) with a capacity report. Our manufacturing capacity is officially monitored by TOBB during our capacity report's currency (Report No: 11/2018)
Quality management in all manufacturing processes is among the most critical issues for users. At Labsert, we implement a process that exceeds recognized international certification standards to ensure global quality and maintain the reliability of results at the highest level. Some key aspects of quality assurance include:
In addition to these conditions, further tests and analyses are performed in accordance with ISO 17034 requirements for reference standards:
All raw materials used in manufacturing are selected for their highest purity, legal compliance, and adherence to standards based on the production field and available supply conditions.
Raw materials for the production of reference standard materials are sourced from reference standard materials and/or high-purity organic compounds. Care is taken to ensure that all solvents used in manufacturing are ultra-pure (Chromatography grade). Amber glass bottles, amber glass ampules, and low-particle HDPE (High-Density Polyethylene) or PPCO (Poly-Propylene Copolymer) bottles undergo bacterial and residual control using suitable wavelength lamps before being put into use.
In the production of QuEChERS kits, pre-tested leak-proof tubes, extra-dried salts, high-purity phase chemicals, and disposable slim salt packs are utilized to guarantee high quality for customers. We also incorporate extra dry and pure sorbent chemicals in the manufacturing of SPE columns.
Temperature and humidity controls are conducted and recorded daily in manufacturing and storage areas. Upon completion of manufacturing, products are prepared for shipment by being placed in appropriate packaging to minimize environmental impact and provide additional protection.
The manufacture of reference standards adheres to conditions that ensure traceability to NIST (when available) and SI units. During instrumental quantitative analysis in our certification process, calibration curves are generated using NIST-traceable certified reference materials. Additionally, calibration is performed with weights that possess NIST traceability for all mass measurements utilized in certification. In cases where NIST traceability is not feasible, we provide similar metrological traceability or traceability through secondary source reference materials.